Every year, millions of prescription drugs move through a complex web of manufacturers, wholesalers, pharmacies, and patients. But somewhere in that chain, a fake pill could slip in-look identical, taste the same, and carry the wrong ingredients. In 2023, the FDA estimated that counterfeit drugs still make their way into the U.S. supply chain, putting lives at risk. The DSCSA was created to stop that.
What Is the DSCSA?
The Drug Supply Chain Security Act (DSCSA) is a federal law passed in 2013 to build a digital track-and-trace system for prescription drugs. It’s not just another regulation-it’s a complete overhaul of how drugs are tracked from the factory to the pharmacy counter. Before DSCSA, each state had its own rules. Some required paper records. Others used different barcode formats. It was a mess. The DSCSA replaced all that with one nationwide standard. The goal? Make sure every prescription drug package can be traced back to its origin. If something goes wrong-a recall, a theft, a fake product-the system can pinpoint exactly which boxes are affected, not entire batches. That saves time, money, and lives.How DSCSA Track-and-Trace Works
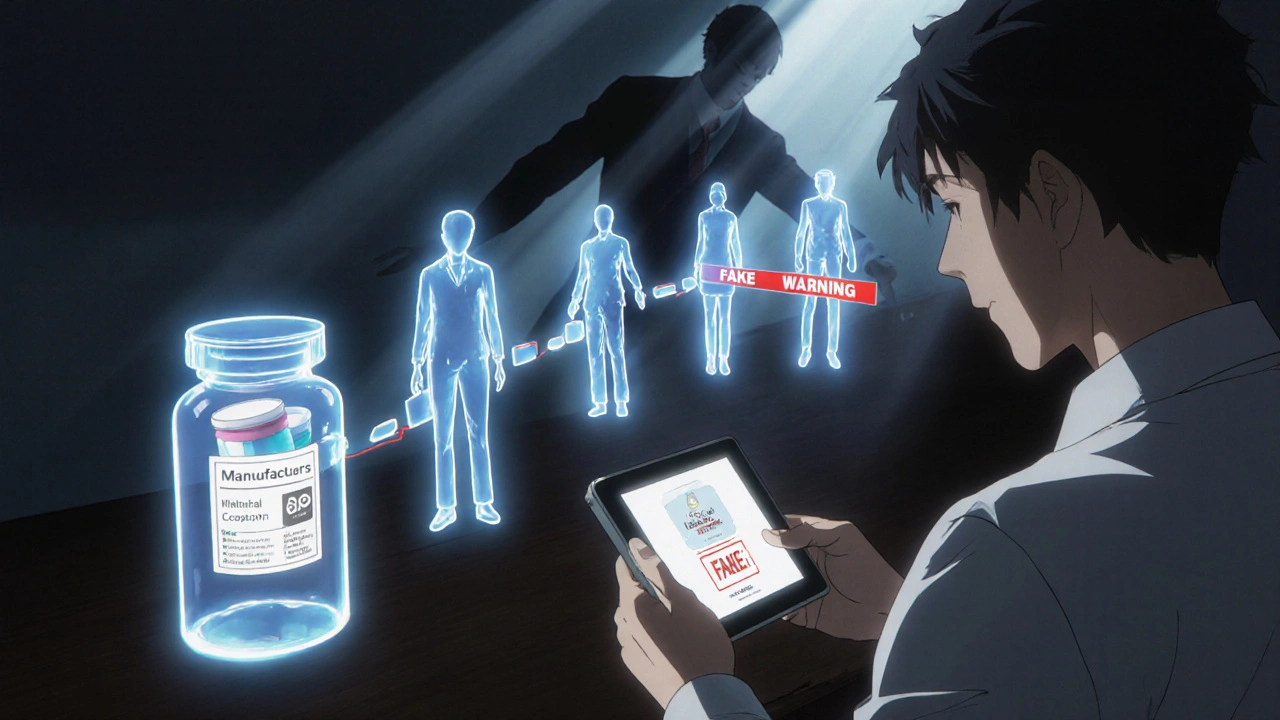
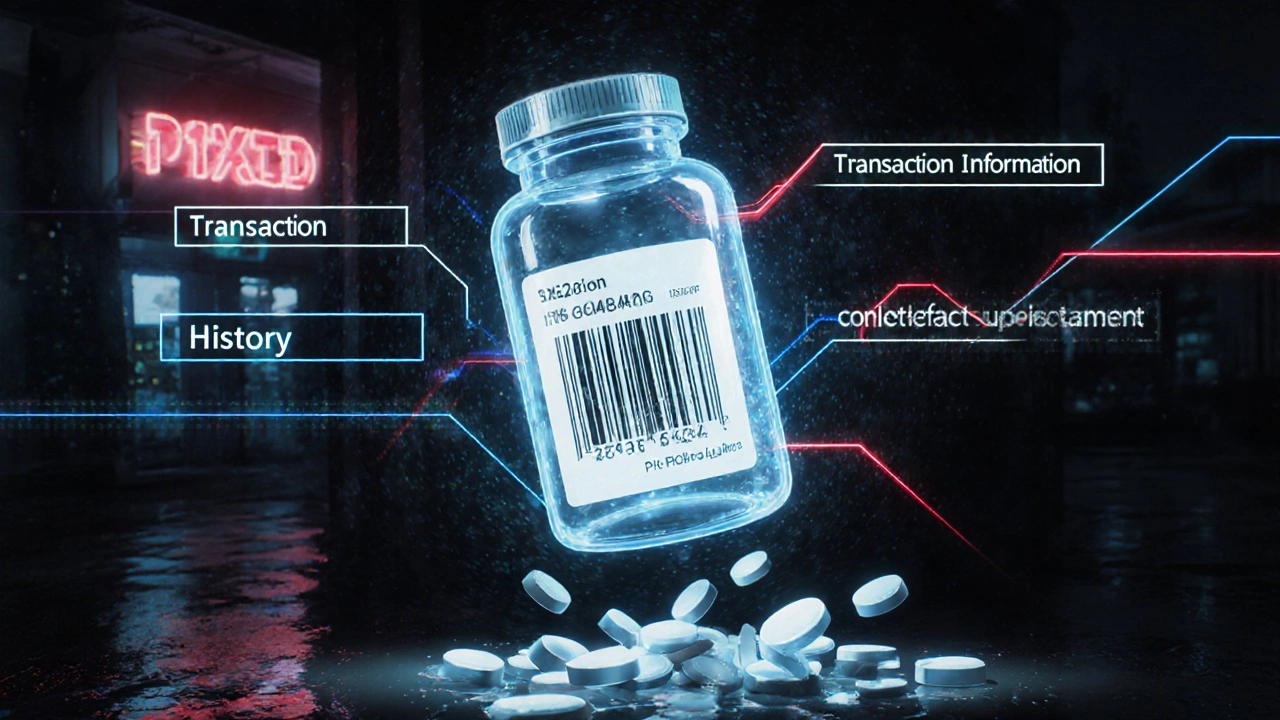
At its core, DSCSA requires every prescription drug package to have a unique identifier. That’s not just the National Drug Code (NDC). It’s also a serial number, lot number, and expiration date-all in a machine-readable 2D barcode and human-readable text. Think of it like a license plate for each pill bottle. When a drug moves from manufacturer to wholesaler to pharmacy, each step must be recorded electronically. Three key pieces of data are shared:- Transaction Information (TI): What’s being shipped, the NDC, serial number, quantity.
- Transaction History (TH): Who handled it before, and when.
- Transaction Statement (TS): A legal certification that the product is legitimate.
Why This Stops Counterfeit Drugs
Counterfeiters don’t just slap on fake labels. They make pills that look real, sometimes even using real packaging. But they can’t replicate the unique serial numbers. Here’s how DSCSA blocks them:- Verification at the point of sale: Pharmacies scan every package before giving it to a patient. If the serial number doesn’t match the manufacturer’s database, the system flags it.
- Impossible to reuse: Each serial number is used once. A fake bottle with a copied barcode will be rejected because the real one already used it.
- Chain of custody: If a box goes missing between a warehouse and a pharmacy, the system shows exactly where it disappeared. That helps catch theft and diversion.
Who Has to Comply?
DSCSA applies to everyone in the prescription drug supply chain:- Manufacturers: Must serialize every package and generate digital transaction data.
- Repackagers: If a company repackages drugs (like putting 100 pills into a bottle for a clinic), they must serialize and trace those too.
- Wholesale distributors: Must verify each shipment before accepting it. They can’t accept products without proper electronic data.
- Dispensers: That’s pharmacies-chain and independent. They must be able to scan and verify each drug package by November 27, 2024.
The Big Deadline: November 27, 2024
The law gave the industry over ten years to get ready. But the final step-full electronic, interoperable, package-level traceability-is now here. By November 27, 2024, every prescription drug in the U.S. must be traceable at the individual package level. No more paper. No more partial systems. No more excuses. The FDA isn’t shutting down the supply chain if things aren’t perfect on day one. They’ve given a one-year stabilization period to work out glitches. But by late 2025, they will start enforcing penalties. That means:- Pharmacies that can’t verify drugs may be forced to stop selling them.
- Distributors who accept unverified products could face FDA warning letters.
- Companies that ignore suspect product reports could lose their licenses.
Challenges and Real-World Problems
It’s not all smooth sailing. Many companies hit roadblocks. One big issue? Data mismatches. A manufacturer sends a serial number in one format. The pharmacy’s system expects it in another. The system rejects the package. The pharmacy can’t stock it. Patients wait. That happened to Walgreens, which spent $120 million on DSCSA upgrades just to fix these errors. Independent pharmacies are especially strained. A 2023 survey by the National Community Pharmacists Association found that 68% of small pharmacies said DSCSA compliance was their biggest technology challenge. The average cost? $185,000 per pharmacy. That’s more than most small pharmacies make in a year. Interoperability is another headache. Not everyone uses the same software. TraceLink, SAP, and Oracle dominate the market, but many smaller vendors don’t speak the same language. That creates delays. Reddit users in r/pharmacy report 2-3 day delays just to verify a shipment.Who’s Doing It Right?
Some companies got ahead of the curve. McKesson, one of the largest distributors, processed over 1.2 billion serialized transactions by 2023 with 99.98% accuracy. Their system caught thousands of suspect products before they reached patients. CVS Health automated their verification process. They didn’t just scan packages-they built AI tools to flag anomalies. Their suspect product investigations dropped by 75%. Even manufacturers like Pfizer and Merck invested heavily in serialization lines. They now produce millions of uniquely tracked packages per day. The difference? They didn’t wait until the last minute. They treated DSCSA like a safety upgrade, not a compliance checkbox.

Comments
Nicole Ziegler
This is actually kind of wild when you think about it 🤯 Like, every single pill has its own license plate now. Feels like sci-fi but it’s real. Glad they’re finally locking this down.
On November 19, 2025 AT 16:59
Bharat Alasandi
DSCSA is the real MVP of pharma compliance. EPCIS integration is a beast but once you’re in, the ROI is insane. Saw a 99.97% verification rate at my firm after we migrated to TraceLink. No more manual checks, no more guesswork. This is how supply chains should operate.
On November 19, 2025 AT 20:32
Kristi Bennardo
This is an absolute disaster waiting to happen. The FDA has no business forcing small pharmacies to spend $200,000 on software they can’t afford. This isn't safety-it's corporate extortion. And don’t get me started on how many systems still can’t talk to each other. This is a bureaucratic nightmare dressed up as progress.
On November 19, 2025 AT 22:43
Shiv Karan Singh
95% reduction? lol. You really believe that? I’ve seen pharmacies still using paper logs in 2024. And who’s auditing this? The same people who let fentanyl laced fake oxycodons slip through last year? This is all theater. The real problem is corruption, not tech.
On November 20, 2025 AT 07:45
Ravi boy
so like the serial numbers are like barcodes but for pills right? and if someone tries to reuse one it just says nope? that sounds smart but also like a lot of work for little pharmacies. i mean i get it but wow
On November 22, 2025 AT 01:53
Matthew Peters
I work in hospital logistics and let me tell you-this system has saved lives. We caught a fake insulin batch last month because the serial didn’t match. The whole shipment got quarantined before it hit the floor. That’s not luck. That’s DSCSA. And yeah, it’s a pain to implement-but imagine if that fake had gone to a diabetic patient. That’s the cost of doing nothing.
On November 23, 2025 AT 06:10
Gerald Cheruiyot
The real question isn't whether the system works but whether we're ready to trust it. We've built this complex digital chain to stop counterfeits but we still rely on humans to input data, to fix errors, to interpret alerts. What happens when the system fails and no one notices? Maybe the real danger isn't the fake pills-it's our blind faith in the machine
On November 24, 2025 AT 17:59
Michael Fessler
Biggest issue i’ve seen is data mismatches. Manufacturer sends NDC in GS1 format, pharmacy system expects HL7. Boom-rejected shipment. Took us 3 months to fix that on our end. And yeah, small pharmacies are getting crushed. $185k isn’t just a cost-it’s a death sentence for some. FDA needs to fund tech grants, not just threaten penalties.
On November 26, 2025 AT 05:14
daniel lopez
This is all a cover-up. The government knows the real counterfeit drugs are coming from inside the system. Big Pharma is using DSCSA to lock out generics and control pricing. The serial numbers? They’re tracking YOU, not just the pills. Your pharmacy scans it, they know what you’re taking. This isn’t safety-it’s surveillance.
On November 26, 2025 AT 18:41
Nosipho Mbambo
I mean... it's a good idea, right? But like... why so expensive? And why now? I don't understand why it's so hard to just... do it. Also, who even reads this? I just want my meds.
On November 27, 2025 AT 00:20
Katie Magnus
So we’re spending millions to track pills but we can’t fix the opioid crisis? This is peak virtue signaling. The real problem is doctors overprescribing. Not barcodes.
On November 29, 2025 AT 00:10
King Over
The system works if you have the money to make it work. The rest of us are just hoping our pharmacist doesn’t miss a red flag while scrolling through TikTok
On November 29, 2025 AT 03:07