Generic drugs are saving billions - but the game is changing fast
By 2024, generic drugs made up 90% of all prescriptions filled in the U.S. - but only 23% of total drug spending. That’s the power of generics: they deliver the same active ingredients as brand-name pills at a fraction of the cost. In many cases, you’re paying 80-85% less. And it’s not just America. Across Europe, Asia, and emerging markets, governments are pushing generics to keep healthcare affordable as chronic diseases like diabetes, heart disease, and cancer keep rising. But here’s the twist: the old model of making cheap small-molecule pills is running out of steam. The real growth is in biosimilars, supply chain shifts, and new manufacturing hubs. If you think generics are just cheaper versions of old drugs, you’re already behind.
Where the money is: biosimilars are the new frontier
For years, generic manufacturers focused on small-molecule drugs - things like metformin for diabetes or atorvastatin for cholesterol. These are chemically simple, easy to copy, and cheap to produce. But now, the biggest patent cliffs aren’t for pills anymore. They’re for biologics: complex, injectable drugs used to treat cancer, autoimmune diseases, and rare conditions. Drugs like Humira, Enbrel, and Keytruda are losing patent protection, and that’s opening the door for biosimilars.
Biosimilars aren’t exact copies. They’re highly similar versions of biologic drugs, made from living cells. That makes them way harder to produce. While a regular generic might take $1-5 million and a few years to develop, a biosimilar can cost $100-250 million and take over a decade. But here’s the catch: they still sell for 15-30% less than the original biologic. That’s not 80% off like traditional generics - but it’s enough to make a huge difference in hospital budgets. In 2024, the biosimilar market grew at 12.3% annually, outpacing all other segments. Companies that stuck with old-school generics are seeing shrinking margins. Those investing in biosimilars? They’re positioning themselves as the next generation of affordable medicine.
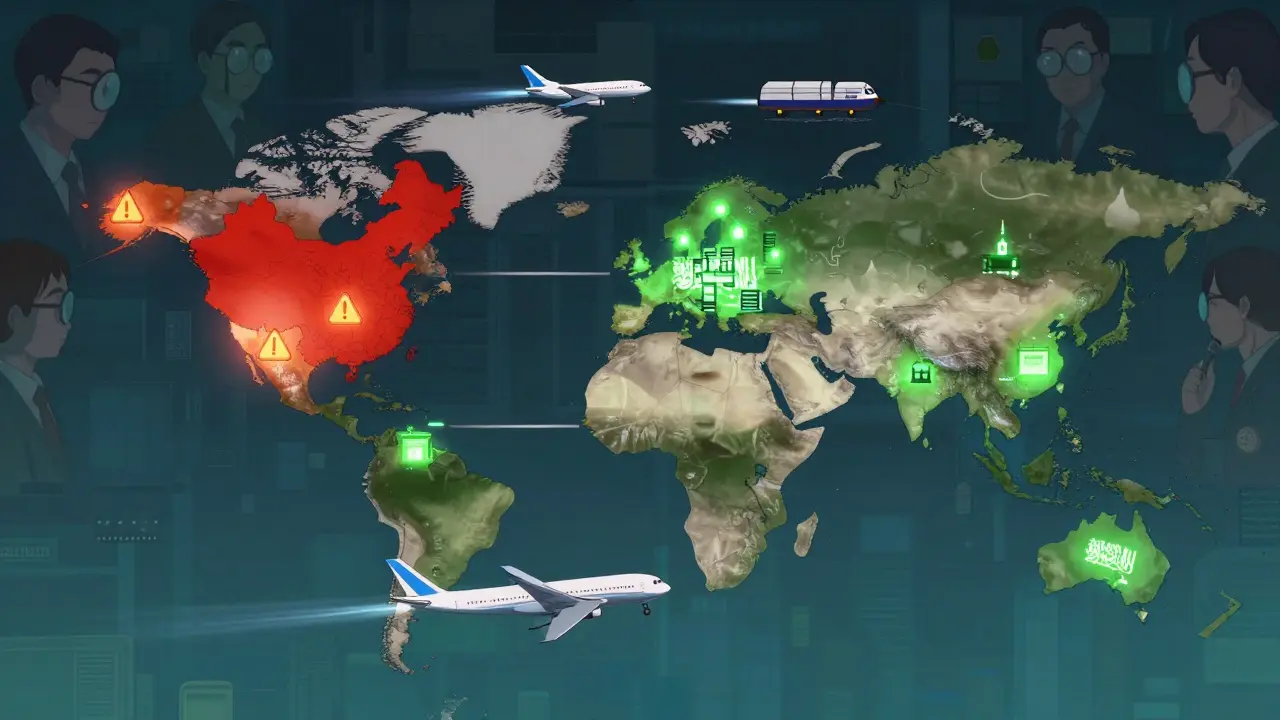
India and China dominate - but the map is shifting
Right now, India makes over 60,000 generic medicines and supplies 20% of the world’s generic volume by volume. China produces about 40% of the world’s active pharmaceutical ingredients (APIs) - the raw building blocks of pills. Together, they control roughly 35% of global manufacturing capacity. That’s not a coincidence. Both countries invested heavily in infrastructure, skilled labor, and regulatory compliance over the last 20 years.
But things are changing. The U.S. and EU are pushing back on over-reliance on China for APIs. In 2024, the FDA issued 187 warning letters to foreign manufacturers - 40% of them linked to quality issues in Chinese and Indian plants. That’s driving governments to fund local production. India’s government poured $1.34 billion into its Production Linked Incentive (PLI) scheme in 2024, aiming to boost domestic API manufacturing. Egypt now requires 50% of essential medicines to be made locally by 2025. Saudi Arabia’s Vision 2030 includes building its own pharmaceutical supply chain. Even the EU is offering subsidies to bring production back home. The era of “Made in India” and “Made in China” as the default is fading. The future belongs to diversified, resilient networks.
Price pressure is squeezing margins - and forcing innovation
Back in 2020, generic manufacturers enjoyed profit margins around 18%. By 2024, that dropped to 12%. Why? More competitors. More price bidding. More government price controls. In Germany, generics are prescribed by default and reimbursed at rock-bottom rates. In Italy, where doctors still favor brands, penetration is just 28%. The gap between rich and poor markets is widening.
But the smart players aren’t just lowering prices. They’re changing their business model. Some are cutting out middlemen and selling directly to hospitals and pharmacy chains. Others are bundling generics with digital tools - like apps that remind patients to take their meds or track side effects. A few are even offering outcomes-based pricing: if the drug doesn’t work as expected, the price drops. It’s not just about selling pills anymore. It’s about proving value. Companies that treat generics as commodities will keep losing. Those that treat them as part of a care system? They’ll thrive.
Regulations are tightening - and that’s good news
For years, the biggest fear around generics was quality. Fake or substandard drugs in Africa and Southeast Asia made headlines. But in 2023, the FDA issued warning letters to 40% of foreign generic plants - not because they were fake, but because their manufacturing processes didn’t meet standards. That’s a sign the system is working. Global regulators are finally aligning. In 2024, 15 more countries joined the International Council for Harmonisation (ICH), meaning a generic drug approved in the U.S. has a better chance of being accepted in Europe or Japan without redoing tests.
This harmonization is a game-changer. It cuts time and cost for manufacturers. It also raises the bar. Smaller companies that used to cut corners can’t compete anymore. Only those with clean, auditable, GMP-certified factories will survive. That’s painful in the short term - but it means patients get safer drugs. And in the long run, it rebuilds trust in generics as a reliable, high-quality option.

The big risk: supply chains still hang on one country
China supplies 65% of the world’s APIs for generic drugs. That’s a massive vulnerability. A single natural disaster, political tension, or export ban could trigger global shortages. We saw this in 2020 with PPE. We saw it again in 2022 with antibiotics. The U.S. government now classifies 125 APIs as critical to national security. Yet, most drugmakers still rely on Chinese suppliers because they’re cheap and efficient.
That’s starting to change. The U.S. Inflation Reduction Act includes funding to rebuild domestic API production. The EU’s Pharmaceutical Strategy aims for 50% self-sufficiency by 2030. India is investing billions to replace Chinese imports. But progress is slow. Building a single API plant takes 3-5 years and over $100 million. The transition won’t happen overnight. But the direction is clear: diversification is no longer optional. It’s survival.
What’s next? The market will shrink - but get smarter
By 2030, generics will still be the backbone of global healthcare. But their share of total drug spending is expected to drop from 57.56% in 2024 to around 53%. Why? Because specialty drugs - like GLP-1 weight-loss drugs, gene therapies, and personalized cancer treatments - are exploding. These aren’t generics. They’re expensive, complex, and hard to copy. They’re capturing more of the spending pie.
But that doesn’t mean generics are fading. It means they’re evolving. The future belongs to companies that can make biosimilars, manage complex supply chains, meet global quality standards, and offer more than just a pill. It’s no longer about being the cheapest. It’s about being the most reliable, the most scalable, and the most integrated into modern healthcare systems.
For patients, that’s good news. More access. Better quality. Lower prices - even as the market gets tougher for manufacturers. For policymakers, it’s a reminder: generics aren’t just a cost-cutting tool. They’re a public health necessity. And their future depends on smart investment, not just cheap labor.
What you need to know in 2026
- Biosimilars are the growth engine - not traditional small-molecule generics.
- India and China still lead manufacturing - but local production is rising fast in Egypt, Saudi Arabia, and the EU.
- Profit margins are shrinking - winners are innovating with service models, not just lower prices.
- Regulations are getting stricter - quality is now non-negotiable.
- Supply chain risks are real - over-reliance on China remains the biggest threat.
- Generics will still dominate prescriptions - but their share of spending will slowly decline as specialty drugs rise.
Are generic drugs as safe as brand-name drugs?
Yes. By law, generic drugs must contain the same active ingredients, dosage, strength, and route of administration as the brand-name version. They must also meet the same strict quality and safety standards set by regulators like the FDA and EMA. The only differences are in inactive ingredients (like fillers or dyes) and packaging - which don’t affect how the drug works. Millions of patients worldwide take generics safely every day.
Why are biosimilars more expensive to make than regular generics?
Biosimilars are made from living cells - like bacteria or yeast - instead of being chemically synthesized like traditional pills. This makes them incredibly complex. A single biosimilar can require 10-20 times more manufacturing steps than a small-molecule generic. Each step must be tightly controlled to ensure consistency. That means expensive equipment, highly trained staff, and years of testing. A regular generic might cost $1-5 million to develop. A biosimilar? $100-250 million.
Can I trust generics made in India or China?
Many are trustworthy - but not all. The FDA inspects foreign manufacturing plants regularly. In 2023, 40% of its warning letters went to facilities in India and China due to quality control failures. That doesn’t mean all drugs from those countries are unsafe. It means you need to look at the regulator’s track record. If a generic is approved by the FDA, EMA, or WHO, it’s been through rigorous checks. Avoid unapproved generics sold online or through unofficial channels.
Why do some countries use more generics than others?
It comes down to policy. In Germany, doctors are required to prescribe generics unless there’s a medical reason not to. Insurance systems reimburse them at low rates, so pharmacies and hospitals have strong incentives to use them. In Italy, doctors still prefer brands, and reimbursement policies don’t push generics as hard. In the U.S., 90% of prescriptions are for generics, but because brand-name drugs are so expensive, generics still make up only 23% of total spending. It’s not about patient preference - it’s about how the system is designed.
Will generic drug prices keep falling?
For simple, old generics - yes, probably. When dozens of companies make the same pill, competition drives prices down. But for newer generics - especially biosimilars - prices are stabilizing. These drugs are harder to make, so fewer companies can enter the market. That means less price pressure. The biggest price drops are behind us. The future is about value, not just cost.
How will the global generic market change by 2030?
By 2030, the market will be smaller as a share of total drug spending - but more sophisticated. Biosimilars will account for a larger slice. Manufacturing will be more spread out, with new hubs in Egypt, Saudi Arabia, and Eastern Europe. Supply chains will be more resilient, though still vulnerable. Regulatory standards will be higher and more aligned globally. Companies that survive will be those that can combine low cost with high quality, innovation, and service - not just cheap pills.

Comments
Sazzy De
Generics saved my dad's life with his blood pressure meds. I never thought twice about it until I read this. Just glad they're still around.
Hope the new hubs don't forget quality while chasing efficiency.
On January 29, 2026 AT 23:58
Gaurav Meena
As someone from India who's seen this shift up close - we're not just making pills anymore. We're building ecosystems. PLI scheme is real. Factories are upgrading. It's not just about cost anymore. It's about trust. And we're earning it. 🙌
On January 31, 2026 AT 23:11
Amy Insalaco
The entire premise is fundamentally flawed. You're conflating volume with value. Generics dominate prescriptions because of perverse reimbursement structures, not because they're inherently superior. The real innovation lies in the biologics and precision medicine ecosystems that generics merely parasitize. The so-called 'evolution' is merely regulatory arbitrage dressed up as progress. The market isn't becoming smarter-it's becoming more extractive under the guise of affordability.
On February 1, 2026 AT 19:13
kate jones
The FDA's warning letters aren't a indictment of India or China-they're a reflection of global regulatory alignment catching up to decades of lax oversight. This isn't protectionism. It's pharmacovigilance. Companies that invested in GMP compliance are thriving. Those that didn't? They're being filtered out. That's market correction, not crisis.
On February 1, 2026 AT 20:44
Kathleen Riley
There is a profound philosophical tension here: the commodification of life-sustaining medicine versus the ethical imperative of accessibility. The market, in its infinite wisdom, reduces human health to a cost-per-dose calculation. Yet, the very molecules that sustain life are now subject to the same supply chain volatility as smartphones. We have optimized efficiency at the expense of resilience. And now, we are relearning that biology does not negotiate.
On February 3, 2026 AT 10:36