Many people assume that if a generic drug is approved, it might be significantly weaker-or stronger-than the brand-name version. Some even think the 80-125% rule means a generic can have 25% less or 25% more of the active ingredient. That’s not true. And this misunderstanding is costing people confidence in medications that save them money and work just as well.
What the 80-125% Rule Actually Measures
The 80-125% rule isn’t about how much active ingredient is in the pill. It’s about how quickly and completely your body absorbs that ingredient. This is called bioequivalence. The U.S. Food and Drug Administration (FDA) uses this range to make sure a generic drug performs the same way in your body as the brand-name version.
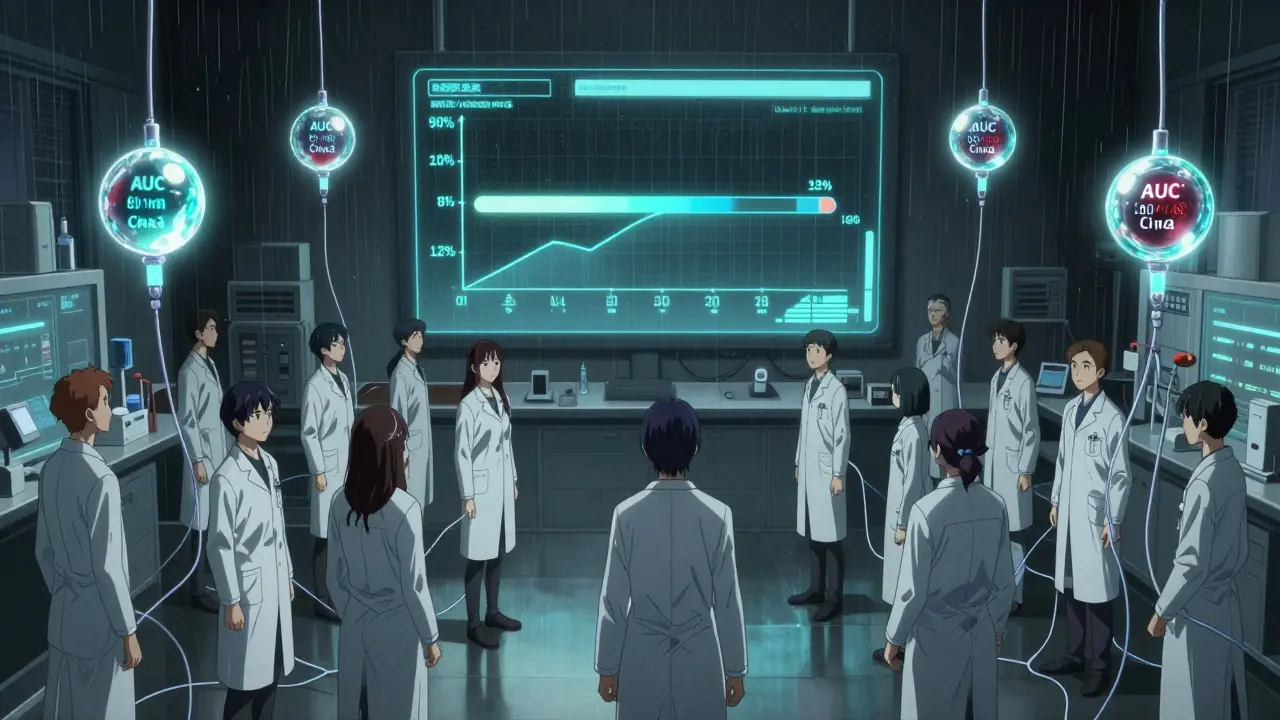
Here’s how it works: Researchers give volunteers both the brand-name drug and the generic version, then measure the drug’s concentration in their blood over time. Two key numbers matter: the area under the curve (AUC), which tells you how much of the drug entered your bloodstream overall, and the maximum concentration (Cmax), which shows how fast it got there.
The 90% confidence interval for the ratio of these numbers between the generic and brand drug must fall entirely between 80% and 125%. That’s not a guess. It’s a strict statistical rule. If even one point of that interval dips below 80% or rises above 125%, the generic fails.
Why 80-125% and not 90-110%? Because drug absorption doesn’t follow a straight line-it follows a logarithmic scale. On that scale, a 20% difference on either side of 100% becomes 80% and 125%. This isn’t arbitrary. It’s based on decades of data showing that differences within this range rarely affect how well a drug works.
What Happens in Real-World Studies
The FDA analyzed over 2,000 bioequivalence studies done between 2008 and 2012. What did they find? Nearly all generics-98%-had actual absorption rates between 95% and 105% of the brand-name drug. That’s far tighter than the 80-125% range suggests.
Think of it like this: If the brand-name drug delivers 100 units of medicine into your blood, the generic likely delivers 98. Or 102. Not 80. Not 120. The 80-125% range is a safety net, not a target.
A 2016 study in JAMA Internal Medicine tracked 2 million patients taking generic or brand-name cardiovascular drugs. No difference in heart attacks, strokes, or deaths. Another study looked at 1,500 patients on generic levothyroxine. Thyroid levels stayed stable. No spikes, no crashes.
Even drugs with narrow therapeutic windows-like warfarin or lithium-have shown no increased risk when switched to generics that meet the bioequivalence standard. The FDA tightened the range to 90-111% for these drugs, and even then, outcomes stayed the same.
Why People Still Doubt Generics
Despite the data, myths persist. Pharmacy students on Student Doctor Network have posted threads with over 15,000 views where people claim generics can have “up to 25% less active ingredient.” Reddit threads show patients refusing generics because they “feel different.”
But here’s the truth: what people feel isn’t always the drug’s fault. Switching from one pill shape or color to another can trigger placebo effects-or nocebo effects. If you believe the generic won’t work, your brain can make you feel like it doesn’t. That’s real. But it’s not the drug.
Pharmacists report that 78% of patients ask about the 80-125% rule at least once a week. And when they’re shown the data-when they hear that the average difference is just 3.5%-most change their minds.
The FDA’s #GenericsWork campaign reached over 1.2 million people. The message? Generics aren’t cheap imitations. They’re scientifically proven alternatives.
How Bioequivalence Testing Works
It’s not easy to prove bioequivalence. A typical study involves 24 to 36 healthy volunteers. Each person takes both the brand and generic versions, in random order, with a washout period in between. Blood is drawn 12 to 18 times over 72 hours. That’s 200+ blood samples per person.
The lab must measure drug levels with precision better than 15% variation. The analysis uses log-transformed data. The confidence interval must be calculated correctly. One mistake-and the whole study fails.
And it’s not just about the numbers. The FDA checks the manufacturing process, the inactive ingredients, the tablet coating, the dissolution rate. All of it matters. A generic isn’t approved just because it has the same active ingredient. It has to behave the same way in your body.
What About Complex Drugs?
Not all drugs are created equal. Inhalers, topical creams, injectables, and some extended-release pills don’t fit neatly into the 80-125% rule. For these, blood levels might not tell the whole story.
That’s why the FDA now has special guidelines for complex generics. For example, with inhaled asthma drugs, they measure lung deposition instead of blood concentration. With topical products, they test skin absorption directly.
These are exceptions. They make up less than 5% of all generic drugs. But they’re important. The FDA is building a database of 1,200 complex drug products to develop better testing methods. The goal? Keep the same high standard, even when the science gets harder.

Why This Rule Matters for You
Generics now make up 90% of all prescriptions in the U.S. They saved the healthcare system $373 billion in 2021 alone. Without the 80-125% rule, none of that would be possible.
This rule isn’t a loophole. It’s a shield. It protects you from unsafe or ineffective products. It ensures that every generic you pick up at the pharmacy has been tested against the same gold standard as the brand.
And it works. The FDA’s Sentinel Initiative, which tracks 200 million patient records, found no increase in adverse events for 94% of generic drugs between 2015 and 2020.
When your doctor prescribes a generic, they’re not cutting corners. They’re trusting science.
What to Do If You’re Still Unsure
If you’ve had a bad experience with a generic, talk to your pharmacist. Ask them to explain the bioequivalence data. Ask if the drug is on the FDA’s list of complex generics. Ask if there’s a reason to stick with the brand.
But don’t assume the generic is the problem. The real issue is often fear-not science.
For most people, generics are not just cheaper. They’re just as good.
Does the 80-125% rule mean generic drugs can have 25% less active ingredient?
No. The 80-125% rule refers to the rate and extent of absorption into your bloodstream, not the amount of active ingredient in the pill. Both brand and generic versions contain the exact same amount of active ingredient. The rule ensures your body absorbs it similarly, not that the pill contains less or more drug.
Why is the range 80-125% and not 90-110%?
Because drug absorption data is log-normally distributed, not linear. A 20% difference on a logarithmic scale translates to 80% and 125%. A fixed 90-110% range would be statistically misleading and could reject generics that are actually equivalent. The 80-125% range, combined with a 90% confidence interval, is mathematically precise and clinically safe.
Are generics as safe as brand-name drugs?
Yes. The FDA’s Sentinel Initiative tracked over 200 million patient records from 2015 to 2020 and found no significant difference in adverse events between brand and generic drugs for 94% of products. Generics must meet the same manufacturing and quality standards as brand-name drugs.
Why do some people feel different on generic medication?
Often, it’s psychological. Changing pill size, color, or brand can trigger nocebo effects-where you expect side effects and feel them. The drug itself hasn’t changed. Studies show that when patients are properly informed about bioequivalence, their concerns drop significantly.
Do all countries use the 80-125% rule?
Yes. The European Medicines Agency (EMA), Health Canada, and over 50 other countries use the same 80-125% bioequivalence standard. The rule is internationally recognized as scientifically valid. Some emerging markets have modified versions for specific drugs, but the core standard remains unchanged.
Can a generic fail bioequivalence testing?
Yes. About 32% of generic applications are initially incomplete or fail bioequivalence testing. The FDA requires strict protocols, and if the 90% confidence interval for AUC or Cmax falls outside 80-125%, the drug is not approved. This is why most approved generics perform within 95-105% of the brand.
Are generics tested on real patients or just healthy volunteers?
Bioequivalence studies are done in healthy volunteers to control variables like disease state or other medications. But the FDA requires post-market monitoring of real patients through systems like the Sentinel Initiative. Real-world data confirms that the results from healthy volunteers predict performance in actual patients.

Comments
Emily Leigh
Okay but like… why does it feel like my generic Xanax makes me feel like a zombie while the brand name makes me feel like a slightly less anxious version of myself? 🤷♀️
On January 18, 2026 AT 16:48
Renee Stringer
The FDA’s standards are not a suggestion. They’re a legal requirement. If you’re still skeptical, you’re not being cautious-you’re being dangerously uninformed.
On January 19, 2026 AT 15:56
Crystal August
Let me tell you what really happened. My cousin took a generic blood pressure med and ended up in the ER. The pharmacist said it was ‘within range.’ That’s not good enough. Who tests these things? Some guy in a lab coat with a calculator?
On January 21, 2026 AT 08:17
Courtney Carra
It’s funny how we trust algorithms to pick our dating matches but don’t trust statistical models to tell us if a pill will work. We’re all just one placebo effect away from becoming modern-day alchemists.
The 80-125% rule isn’t arbitrary-it’s the mathematical embodiment of human biology’s messy, logarithmic truth. We want black-and-white answers, but medicine? It’s shades of gray… with confidence intervals.
And yet, we still panic because the pill is a different color. We’re terrified of the unknown, even when the data is screaming at us.
It’s not about the drug. It’s about control. We want to believe we can see the difference. But sometimes, the difference is just in our heads.
And yes-I’ve taken generics for 12 years. No crashes. No spikes. Just… relief. At a third of the price.
On January 22, 2026 AT 20:03
thomas wall
The notion that a 25% variance in absorption is acceptable is, frankly, a scandal. In the UK, we demand tighter tolerances. The FDA’s standard is a relic of American deregulation culture. Patients deserve better.
On January 23, 2026 AT 05:11
pragya mishra
My brother works at a generic drug factory in India. He says they use cheaper fillers, skip tests, and sometimes mix batches from different plants. You think the FDA checks every single pill? Wake up. This is capitalism, not science.
On January 24, 2026 AT 19:59
Manoj Kumar Billigunta
Hey, I get it. Change is scary. But generics saved my mom’s life. She couldn’t afford the brand, so she tried the generic for her diabetes meds. Her numbers stayed perfect. No side effects. No drama. Just good science and lower bills.
Don’t let fear stop you from doing what’s smart. Talk to your pharmacist. Ask questions. But don’t throw out the science because the pill looks different.
On January 25, 2026 AT 13:15
Andy Thompson
98% of generics are fine? LOL. That’s just the number they want you to see. What about the 2% that slip through? The ones that cause seizures? The ones that make people suicidal? They don’t publish those stats. Big Pharma and the FDA are in bed together. You think they care if you live or die?
On January 26, 2026 AT 04:47
sagar sanadi
So the rule is 80-125% but they’re all actually 95-105%? So why even have the rule? Just to make people feel like they’re getting a bargain while the government lets them take a gamble? Classic.
On January 26, 2026 AT 15:01
kumar kc
Generics work. Stop complaining.
On January 28, 2026 AT 01:08