When you pick up a prescription, you expect it to be safe, genuine, and properly handled. That’s not just luck—it’s thanks to the DSCSA, the Drug Supply Chain Security Act, a U.S. federal law designed to build an electronic, interoperable system to trace prescription drugs from manufacturer to pharmacy. Also known as the Drug Traceability Law, it’s the backbone of modern drug safety in America. Before DSCSA, fake or contaminated drugs could slip through the system unnoticed. Now, every package has a unique identifier, and every handoff is recorded digitally. This isn’t just paperwork—it’s a shield against counterfeit opioids, stolen insulin, and tampered cancer drugs.
DSCSA doesn’t just apply to big pharma. It affects every pharmacy, distributor, and wholesaler in the chain. If you work in a community pharmacy, you’re scanning barcodes at pickup. If you’re in a hospital, you’re verifying serial numbers before giving meds to patients. The law requires companies to exchange transaction information electronically by 2023, and by 2025, all prescription drugs must be fully traceable at the package level. That means if a recall happens, you can find exactly which bottles are affected—down to the individual unit—instead of pulling entire shipments off shelves.
Related to DSCSA are the tools and systems that make it work: serialization, blockchain-like ledgers, and interoperable data platforms. These aren’t buzzwords—they’re the actual tech pharmacies use every day to comply. You’ll find posts here that explain how these systems prevent duplicate therapy errors, stop early refills from counterfeit sources, and even tie into FDA label updates that warn about recalled batches. It’s not theoretical. A pharmacy in Ohio recently caught a fake version of a blood pressure med because DSCSA flagged the serial number. That’s the kind of real-world protection this law delivers.
What you’ll find in this collection are clear, practical guides on how DSCSA shapes daily pharmacy work, what changes you need to watch for, and how it connects to other drug safety rules like boxed warnings, generic drug verification, and supply chain transparency. No jargon. No fluff. Just what matters when you’re holding a prescription in your hand—or filling one for someone you care about.
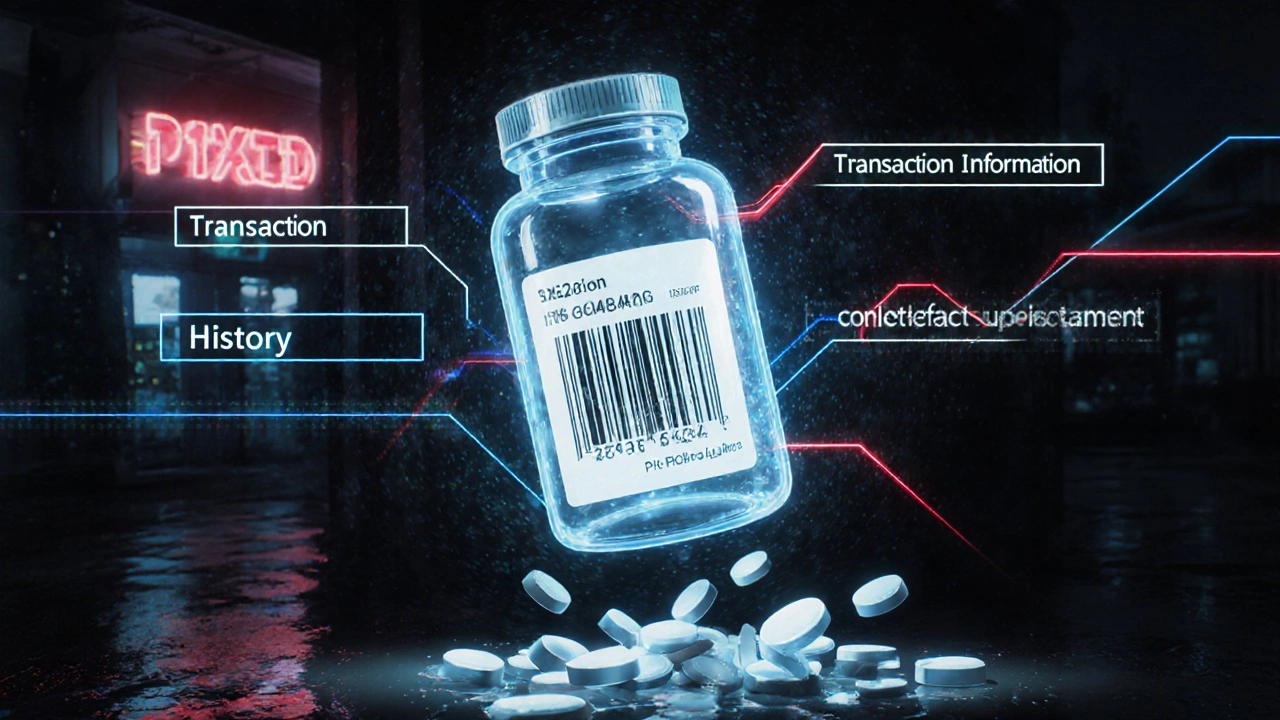
The DSCSA track-and-trace system is the U.S. government's federal solution to stop counterfeit drugs by requiring every prescription package to have a unique digital identifier. Learn how it works, who must comply, and why it matters for patient safety.
Read More© 2026. All rights reserved.