When you take a prescription drug, you're trusting that the FDA drug safety, the U.S. Food and Drug Administration's system for monitoring and communicating risks of medications. Also known as pharmaceutical safety oversight, it's the backbone of how we know if a medicine is truly safe for long-term use. But here’s the thing: FDA drug safety isn’t just about approval. It’s about what happens after the pill hits the shelf. The FDA doesn’t stop watching once a drug is sold. It keeps tracking side effects, new dangers, and hidden risks through real patient data—and updates labels when needed.
One of the strongest tools in this system is the boxed warning, the most serious safety alert the FDA can require on a drug label. Also known as black box warning, it’s printed in bold, surrounded by a box, and can’t be ignored. These warnings show up when a drug carries a risk of death, severe injury, or life-altering side effects—like liver failure, heart rhythm problems, or suicidal thoughts. And they don’t stay the same. Drugs like antidepressants, diabetes meds, and painkillers have had their boxed warnings updated over time as more patients reported harm. That’s why checking FDA label updates, official changes to drug prescribing information published by the FDA isn’t just for doctors. If you’ve been on a medication for years, a new warning could change how you use it.
Then there’s the gap between clinical trials and real life. Clinical trials test drugs on a few thousand people under strict conditions. But once millions start taking it—people with other diseases, on other meds, over 65, or with liver problems—that’s when the real risks show up. That’s why the FDA SrLC database, the FDA’s Safety and Risk Management Database tracking post-market drug safety signals matters. It’s where reports of fainting, liver damage, or sudden confusion from a drug get logged. These aren’t guesses. They’re real patient stories that lead to label changes.
And it’s not just about warnings. The FDA also tracks how drug labels differ from other countries—like the EMA in Europe—because language, risk communication, and even pregnancy warnings can vary. A drug labeled safe in one place might carry a stronger alert elsewhere. Knowing this helps you ask better questions when your doctor prescribes something new.
Below, you’ll find clear, no-fluff guides on how to spot these changes, what the strongest warnings mean, why some drugs get pulled from shelves, and how to use the FDA’s own tools to stay safe. No jargon. No theory. Just what you need to know to protect yourself and your family.
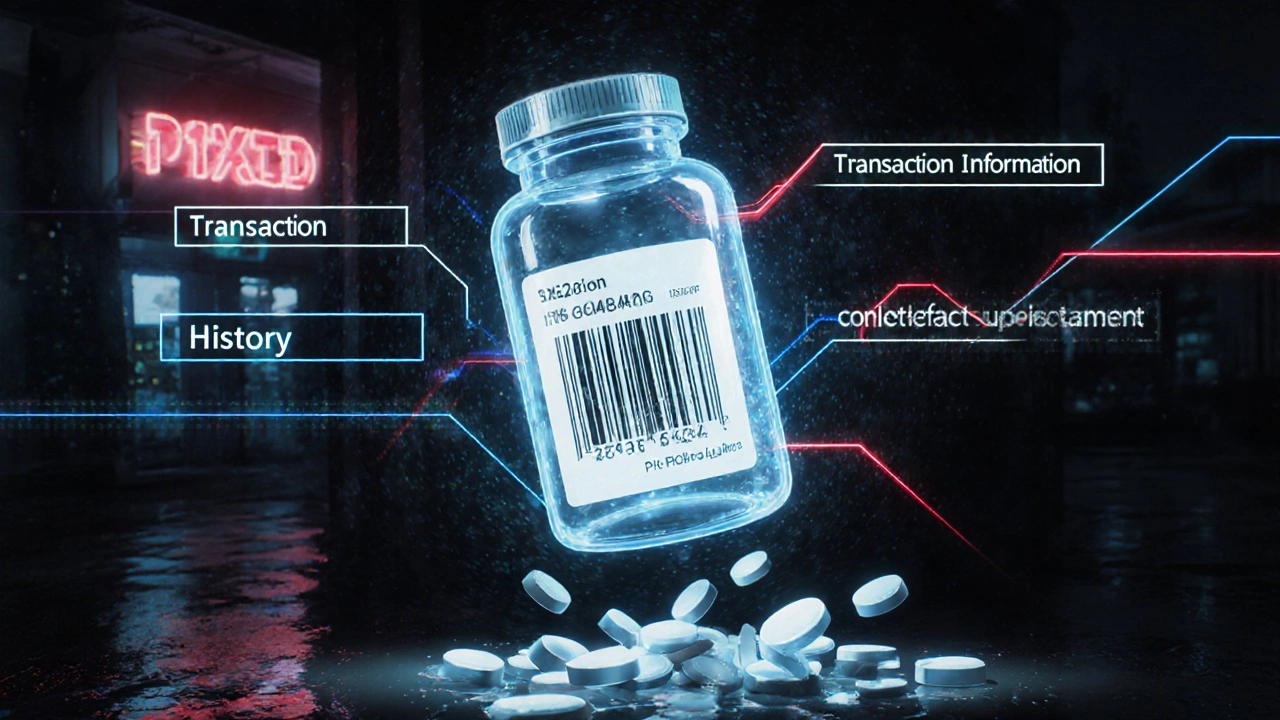
The DSCSA track-and-trace system is the U.S. government's federal solution to stop counterfeit drugs by requiring every prescription package to have a unique digital identifier. Learn how it works, who must comply, and why it matters for patient safety.
Read More© 2026. All rights reserved.