When you pick up a prescription, you’re holding the end result of a pharmaceutical supply chain, the complex network that moves drugs from raw ingredients to patients, involving manufacturers, distributors, regulators, and pharmacies. Also known as the drug distribution system, it’s not just about shipping pills—it’s about keeping them safe, affordable, and available when you need them.
This system touches everything from generic medications, the affordable versions of brand-name drugs that make up most prescriptions to FDA regulations, the rules that control how drugs are tested, labeled, and tracked. You might not think about it, but delays in this chain can mean your medicine isn’t on the shelf. Shortages happen because of factory shutdowns, raw material shortages, or shipping bottlenecks. And when a drug gets recalled, it’s because something went wrong upstream—maybe a contamination in a foreign lab, or a labeling error that missed a critical warning.
The pharmaceutical supply chain isn’t just a pipeline—it’s a web of decisions. A drug made in India, packaged in Germany, shipped through the U.S., and sold in your local pharmacy? That’s normal. But each step adds risk: temperature control during transport, counterfeit drugs sneaking in, or insurance rules forcing pharmacists to switch brands without telling you. That’s why tracking drug manufacturing, where and how medicines are actually made matters. A boxed warning on your pill? That often comes from real-world data collected after the drug left the factory. A generic version that doesn’t work like the brand? That could be a dissolution profile issue—something tested in a lab but never checked once it hit the shelf.
What you’ll find below are real stories from people who’ve been caught in the cracks. One person got a different pill because their pharmacy switched suppliers without warning. Another couldn’t refill their kidney medication because the phosphate binder was on backorder. Someone else took a drug with a new boxed warning because the label update never reached their doctor. These aren’t rare cases—they’re symptoms of a system that’s stretched thin.
There’s no magic fix. But understanding how drugs move—from the lab to your hand—helps you ask better questions. When your prescription changes, ask why. When you see a price jump, ask where the cost went. And when a drug disappears from the shelf, know it’s not just bad luck—it’s a chain reaction you can learn to anticipate.
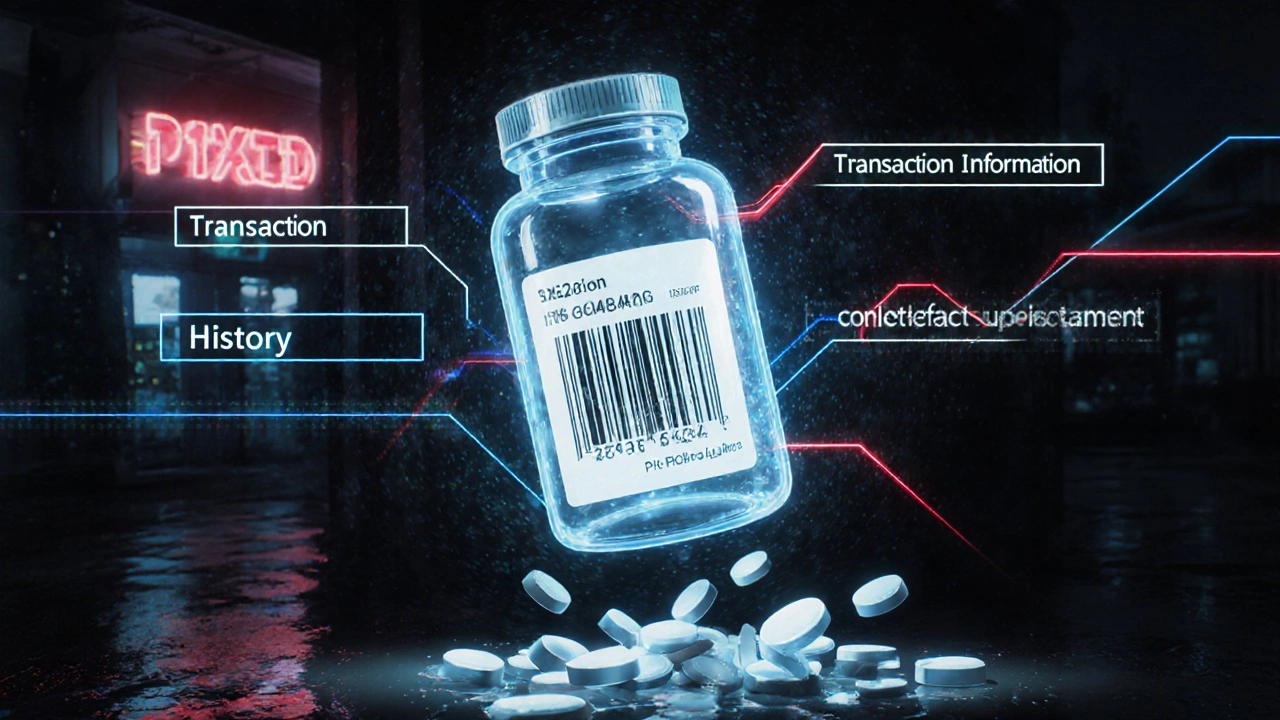
The DSCSA track-and-trace system is the U.S. government's federal solution to stop counterfeit drugs by requiring every prescription package to have a unique digital identifier. Learn how it works, who must comply, and why it matters for patient safety.
Read More© 2026. All rights reserved.